COVID-19 Vaccines and Therapeutics: What You Need to Know
This article is part of an ongoing series on COVID-19, a collaboration of the Colorado School of Public Health, the Denver Museum of Nature & Science, and the Institute for Science & Policy. Tune in to our next webinar on Monday, May 18 at 8:30 a.m. MT discussing stories from front line medical caregivers. Find all of our previous COVID-19 webinars and recaps here.
The search for an effective COVID-19 treatment has commanded the attention of policymakers and the public in recent months, with much speculation around potential vaccines and therapeutics. To understand how and why such treatments might work, immunology is an important starting point. Think of your immune system as a highly trained army, with different components specializing in different types of responses. We’ll walk through them generally and then see how they apply to COVID-19 specifically.
Your innate immune system, or general resistance system, is your first line of defense. These are the body’s anatomical barriers, from your skin to the tiny cilia inside your lungs to the acidity of your stomach to the mucous membranes inside your mouth. Fevers, for example, are your body’s physiological attempt to kill intruders with excess heat. These systems help prevent viruses and other things from entering the body and taking hold.
The other aspect of your immune system is called the adaptive immune system, which spurs actions specific to a particular infection. Antibodies are chemicals that can recognize a germ and either help kill it directly or just tag it to identify it as a target for the rest of the immune system to train its firepower on.
We’ve heard a lot about a COVID-19 vaccine, but even that term itself is multi-faceted. Most people are pretty familiar with live vaccines, which take a weakened form of the germ and inject it into you so that your immune system will learn to recognize it and respond to it. This is your typical measles/mumps/rubella vaccine, the chickenpox vaccine, and so on. Although live vaccines work incredibly well, they require lots of safety testing and typically are unable to be made in a very short period of time.
The other type of vaccine is called an inactivated vaccine, which uses a killed version of the disease (we see this use for influenza, hepatitis A, and more). These often require multiple inoculations over multiple years. And finally, we have seen some genetically engineered vaccines in recent years, which mimic the genetic makeup of a virus and then deliver instructions to your immune system to identify and attack it without having to inject any of the actual virus. Unfortunately, no genetic vaccines have been licensed for human use to date.
Passive immunization ─ in which plasma from a recovered COVID-19 patient could be transfused into another infected person ─ could be another option. In theory, the transfer of antibody-rich convalescent plasma could boost the recipient’s immune system enough to mitigate the disease. (A version of this process occurs naturally during childbirth, when a mother conveys maternal antibodies to her child via the placenta.) However, the exact nature of COVID-19 antibodies and to what degree they are convey immunity are still under investigation.
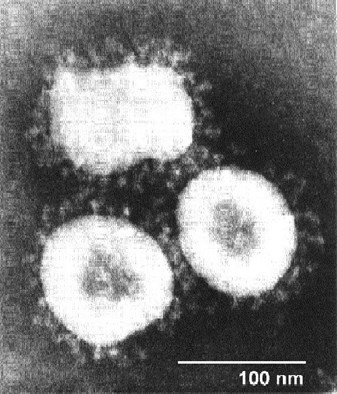 We need to look for a unique marker, something that the immune system can recognize and target. As you can see under an electron microscope, the corona, or crown, spikes have proteins in them that are very specific to COVID-19 and that might be a good place to start. You want to find something that you're going to see every time in the virus.
We need to look for a unique marker, something that the immune system can recognize and target. As you can see under an electron microscope, the corona, or crown, spikes have proteins in them that are very specific to COVID-19 and that might be a good place to start. You want to find something that you're going to see every time in the virus.
All of this underscores the challenges of developing a COVID-19 vaccine on a short timescale. The vaccine development landscape really runs the gamut right now, with lots of labs testing lots of different approaches. Some are creating killed vaccines, some of them are live. Some of them will require multiple doses, some of them would be a single dose. With all the moving parts and the high safety thresholds in place, the notion of having something in place by the fall seems unrealistic. If we get very lucky, we might be able to have one in about a year.
In the interim, therapeutics for COVID-19 could help with improving patient treatment and outcomes. Therapeutics are drugs or other types of biological compounds that can be used to treat people who already have the infection, with the goal of getting them better quicker and preventing death.
One of the things we know about COVID-19 is that it's very different in different people. On one end of the spectrum, some people may be asymptomatic. Other people may have some mild symptoms, like a bad cold or a case of the flu. Most will recover on their own without ever needing treatment. On the other end of the spectrum there is severe and critical illness that's bad enough to warrant hospitalization. Therapeutics could, in theory, keep people from progressing along this spectrum of illness so that they can recover sooner.
There are some fundamental challenges in developing any treatment for COVID-19, however. We need to have randomized controlled trials with large numbers of participants and a control group for comparison. Blinding treatment assignments help reduce bias and errors ascribed to the placebo effect.
There are two broad categories of therapeutics: antivirals and immune modulators. We already use antivirals for many other infections including HIV and hepatitis C infection. We even have an antiviral used to treat common influenza. And so, it leads us to believe that this is a potentially viable strategy for treating COVID-19. There has been lots of talk around the use of particular anti-inflammatory agents, including hydroxychloroquine ─ commonly used to treat malaria, lupus, and rheumatoid arthritis ─ as well as remdesivir, which specifically blocks the enzyme that replicates the viral genetic material. Immune modulators work to dampen the body’s overactive immune responses; several including plasma are currently under study.
The National Institutes of Health has created coronavirus treatment guidelines in which a panel of experts takes a close look at the literature surrounding various potential treatments and then evaluates the evidence in two ways. It found insufficient clinical data to recommend either for or against:
Hydroxychloroquine/chloroquine
Remdesivir
Convalescent plasma
Interleukin-6 inhibitors
And strongly recommends against:
Hydroxychloroquine + azithromycin (AIII)
Lopinaivr/ritonavir (AI)
But fortunately, clinical trials evaluating treatments for COVID-19 are progressing rapidly and this past week was a big one for therapeutic news:
On April 27, the pharmaceutical company Regeneron announced that sarilumab was not effective in people with severe COVID-19, but might provide benefit in some cases
On April 29, the NIH and the pharmaceutical company Gilead Sciences announced that an NIH study found that remdesivir shortens time to recovery in severe COVID-19 patients
On May 1, the FDA granted Emergency Use Authorization for remdesivir
Remdesivir may or may not end up being the most effective medicine for treating COVID-19, but it certainly looks like it's going to be the first to see widespread distribution. We foresee a long road ahead, but stress the importance of conducting very well designed clinical trials.
Lightning Round: Questions from Viewers
What can I do to boost my immunity?
The best advice we can give is to live a healthy life. Exercise regularly, eat good foods, and eat a diet rich in fruits and vegetables which contain antioxidants and anti-inflammatory compounds. And if you have underlying medical issues, make sure that you're managing those properly so that they don't get out of control and leave you more vulnerable than necessary.
What are your thoughts on herd immunity for COVID-19?
There is significant hope that herd immunity will help decrease the incidence of COVID-19 in the future, but the extent of herd immunity in the community is still unknown.
Do people who are asymptomatic still develop antibodies?
This is a great question, but we don’t have an answer to this yet.
Is there a risk with rushing a COVID19 vaccine to market?
As will all treatments, there needs to be sufficient data on safety and efficacy to ensure that the treatment or prevention does not cause harm.
Do we know if the virus will mutate?
So far we have not observed much mutational change in the virus since it entered humans in late 2019. Whether or not the virus will evolve as it continues to spread around the world, we do not know and this will need to be monitored.
Disclosure: Dr. Campbell has previously received consulting fees from Gilead Sciences, ViiV, Merck, and Janssen in addition to research support from Gilead Sciences and ViiV.

Medical Director of Infection Control at UCHealth University of Colorado Hospital

Professor of Medicine and Microbiology at the University of Colorado School of Medicine
Disclosure statement:
The Institute for Science & Policy is committed to publishing diverse perspectives in order to advance civil discourse and productive dialogue. Views expressed by contributors do not necessarily reflect those of the Institute, the Denver Museum of Nature & Science, or its affiliates.
